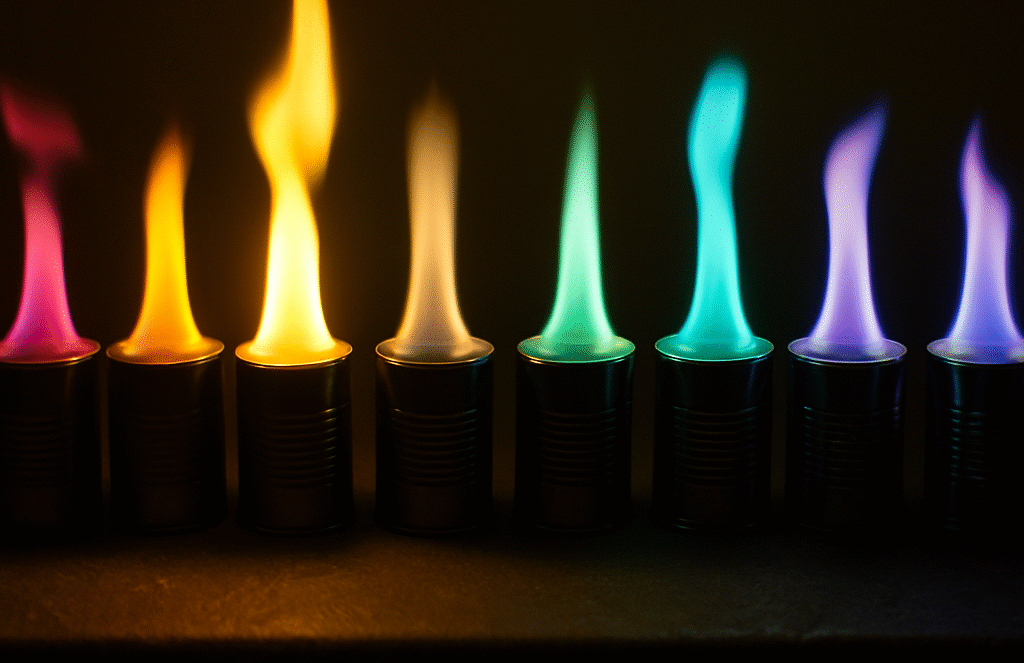Table of Contents
The “Rainbow Flame Experiment” is a visually stunning demonstration often used to capture students’ interest in chemistry. By burning metal salts, each producing a different colored flame, teachers can explain concepts like electron excitation and emission spectra. But beneath its vibrant display lies a combustible danger—one that has burned students, destroyed trust, and sparked legal action.
When Science Dazzles — and Destroys
In too many classrooms across America, excitement has come at the cost of safety. The repeated misuse of methanol, a volatile and highly flammable substance, has turned classrooms into emergency rooms. One such case changed everything: the story of Calais Weber, a student permanently injured by the Rainbow Experiment, and the focus of the powerful safety video After the Rainbow by the U.S. Chemical Safety and Hazard Investigation Board (CSB).
This article examines what went wrong, why it keeps happening, and what educators, administrators, and parents must do to make science both inspiring and safe.
The Incident: A Moment of Flame, a Lifetime of Consequences — After the Rainbow
In 2006, Calais Weber, a high school student in Ohio, was excited to witness a colorful chemistry demonstration. Her teacher, like many others, used methanol to help metal salts ignite in a blaze of rainbow-colored flames. When the fire dimmed, the teacher added more methanol from a bulk container—without a fume hood, without proper safety barriers, and without warning.
The fire flashback was instantaneous. A near-invisible vapor trail ignited, causing a fireball that engulfed Calais and several classmates. She suffered third-degree burns over 40% of her body, spent weeks in a medically induced coma, and underwent numerous surgeries and physical therapy sessions.
This was not an isolated incident. In the years since, similar methanol-fueled experiments have caused injuries at schools, museums, and science events. In some cases, the victims were as young as four years old.
The Science Behind the Risk: Why Methanol is Dangerous
Methanol is a clear, colorless, and highly flammable liquid. Its vapors are heavier than air and can travel across surfaces unnoticed—making it especially dangerous when poured near open flames. Unlike ethanol, which burns with a yellow-orange flame, methanol burns with a nearly invisible blue flame, giving little warning of its presence.
When teachers refill a container mid-experiment or pour methanol from a bulk source, they risk igniting a vapor trail back to the container—causing what’s known as a flashback fire. This fire can explode outward, unpredictably and with devastating force.
Add in a classroom full of students, a lack of protective barriers, and little PPE, and the risk multiplies exponentially.
Who’s Responsible? The Duty of Care in School Laboratories
The concept of “duty of care” applies directly to education professionals. It is the moral, legal, and professional obligation to ensure students are not placed in harm’s way. In the case of the Rainbow Experiment, this duty was violated in several key areas:
1. The Teacher’s Role
Teachers must be trained not just in the science content but in hazard identification, risk mitigation, and emergency response. They are responsible for:
- Conducting written risk assessments
- Understanding chemical properties (e.g., volatility of methanol)
- Using small quantities only and storing chemicals properly
- Wearing and requiring PPE (goggles, gloves, coats)
- Demonstrating only in ventilated environments or fume hoods
- Never pouring additional fuel on a flame
2. Administrative Oversight
School leaders must ensure:
- Teachers are properly trained in lab safety protocols
- Schools maintain a Chemical Hygiene Plan (CHP)
- Proper lab infrastructure (fume hoods, fire blankets, emergency showers) is in place
- A culture of safety, not shortcuts, is rewarded
- Incident reporting systems are accessible and active
3. District-Level Responsibility
Districts and boards of education are accountable for:
- Funding science safety training and equipment
- Hiring qualified Chemical Hygiene Officers (CHOs)
- Requiring regular safety audits
- Supporting professional development in science instruction and lab compliance
4. Student and Family Role
Students must be:
- Educated in basic lab safety principles
- Encouraged to speak up if they feel unsafe
- Given clear instructions and expectations before any experiment
Parents should have access to school safety policies and feel empowered to ask questions about chemical demonstrations or experimental procedures.
The Legal Consequences: When Accountability Fails
When a teacher or school fails in their duty of care, the consequences aren’t just physical—they’re legal. In one high-profile case in New York, a student severely burned during a methanol-fueled Rainbow Experiment received a $59 million settlement after suing the school district for negligence.
Other incidents have led to lawsuits, teacher suspensions, and sweeping policy changes. But the real price is paid by the students whose lives are forever altered.
Alternatives to the Rainbow Experiment: Safer Science, Same Wonder
The scientific principles behind the Rainbow Flame Experiment can be demonstrated safely. Leading safety organizations like the American Chemical Society and CSB recommend alternatives such as:
- Burning pre-soaked wooden sticks in Bunsen burners, using metal salt solutions in minimal quantities
- Performing experiments under fume hoods with remote ignition
- Virtual simulations and video demonstrations for high-risk reactions
- Using LED flame color boards to visually represent flame colors without real combustion
These alternatives maintain the educational value while removing unnecessary risk.
After the Rainbow: Lessons That Must Be Learned
The CSB’s video After the Rainbow doesn’t just chronicle Calais Weber’s story—it serves as a moral imperative. Every science educator should watch this film and reflect: Are my practices safe enough? Have I prioritized spectacle over student well-being?
“I had a plan for my life,” Calais says in the video, “and that plan was taken away from me in an instant.”
Her words are haunting—but they should be galvanizing. No child should be permanently injured in the pursuit of learning.
A Call to Action: What Schools Must Do Today
- To prevent another tragedy, schools must take decisive action:
- Eliminate the use of bulk methanol in classroom experiments
- Require safety training certification for all science educators
- Invest in infrastructure: fume hoods, eyewash stations, fire extinguishers
- Designate a Chemical Hygiene Officer in every building
- Communicate openly with families about lab safety protocols
- Mandate written safety protocols for any live flame or chemical demo
- Encourage a culture of safety-minded curiosity, not unsafe showmanship
Protecting Our Students Is Non-Negotiable
Education should inspire—not injure. Lab experiments have the power to ignite passion for science, but that ignition must always be metaphorical, not literal. The Rainbow Experiment, when performed carelessly, is a ticking time bomb. But when approached with respect, responsibility, and the right safety practices, it can be a teachable moment—for all the right reasons.
Let “After the Rainbow” not just be a title—but a turning point.
After the Rainbow – USCSB
Subscribe to edCircuit to stay up to date on all of our shows, podcasts, news, and thought leadership articles.